This article is for informational purposes only and does not diagnose any conditions
Age-related macular degeneration (AMD) is one of the leading causes of irreversible blindness in the world. Although it currently has no cure, there are multiple things that a person can do to help prevent and treat this condition.
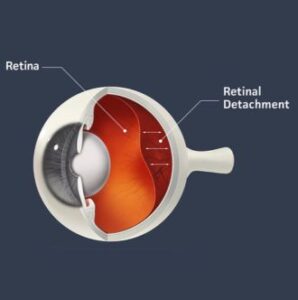
Age-related macular degeneration (AMD) is a retinal condition that primarily affects the retina, which is like film in a camera. Specifically, it damages the macula, which is where our sharp central vision resides and spares the peripheral retina which is involved in side vision. The macula enables a person to perform activities such as reading fine print and other tasks that require excellent vision such as recognizing faces, reading a book, or watching television.
In AMD, the cells in the macula become damaged and the macula degenerates. Because the risk of developing AMD increases with age, macular degeneration is considered, at least in part, a disorder of aging. Together, these three factors are what give the condition its name, i.e. age-related macular degeneration, or AMD.
Before we can understand how AMD affects the retina, it is important to have some sense of normal retina structure and function.
AMD is a disease affecting several layers of the retina including the photoreceptors (the actual film in the camera), retinal pigment epithelium (RPE)(nourishes and removes by products from the photoreceptors), Bruch’s membrane, and the choroid (blood supply to the outer retina). Where degeneration begins and why it starts are questions that still do not have concrete answers.
However, there are some features of AMD that are relatively consistent across patients and include the development of Drusen in early stages, and in late stages—geographic atrophy and/or choroidal neovascularization.

Drusen Levels Can Indicate Early AMD
The hallmark of early AMD is the presence of drusen. Drusen, from the German word for node, are deposits of extracellular material lodged between the RPE and Bruch’s membrane. [12-15] Most people over the age of 40 have a few small drusen which does not represent AMD. However, drusen in larger numbers and larger size are a sign of AMD.
Drusen, the German word for node, are deposits of extracellular material lodged between the RPE and Bruch’s membrane. [12-15] Most people over the age of 40 have a few small drusen. However, drusen in larger numbers and larger size often is a sign of AMD.
WHAT ARE THE DIFFERENT TYPES OF DRUSEN?
Drusen appear as yellow or white spots on the retina and come in two overarching categories: hard and soft. Hard drusen have well-defined borders and are usually small, while soft drusen are larger with indistinct borders that can grow in size, sometimes filling with cholesterol or becoming calcified over time. [12] Although a few small, hard drusen typically develop through normal aging processes, having numerous or medium-sized drusen in the macula is usually a sign of early to intermediate AMD. Drusen by themselves do not necessarily cause extensive declines in visual acuity, although depending on their severity, they can cause distortions in central vision and affect a patient’s sensitivity to color and contrast. [12] This is because the concentration of cone photoreceptors (responsible for color and fine vision) is highest in the macula. [16] Therefore, any abnormalities in the macula have the potential to lead to distortions in color vision as well as declines in the ability to see fine details.
GEOGRAPHIC ATROPHY A FEATURE OF DRY AMD
Geographic atrophy is a feature of late-stage dry AMD, where RPE cells have died and the overlying photoreceptor layer has begun to break down as well. [14, 18] Usually, geographic atrophy develops in an area where there previously were numerous or large drusen that have regressed, leaving cell death in their wake. [19] It often develops in a bilateral fashion, meaning both eyes are affected. Regions of the retina that have progressed to this stage usually have very poor visual acuity, since the loss of photoreceptors is devastating to normal visual function. However, the centermost part of the macula, or the fovea, is often spared until late-stage disease. [13] This is fortunate, since the fovea is the spot where visual acuity is the highest, and thus, central vision may be preserved until late in the disease.
CHOROIDAL NEOVASCULARIZATION (CNV)
The choroid is the region of the eye which supplies blood to the retina. Wet AMD is characterized by the formation of new blood vessels in the choroid, known as choroidal neovascularization (CNV). Neovascularization simply means new blood vessel growth — “neo”, means ‘new,’ and ‘vascularization’ refers to blood vessels. CNV refers to the process of new blood vessel formation from the choroid.
Drusen can cause breaks in Bruch’s membrane, through which CNV can grow. [14] The walls of these new blood vessels are often weak and prone to leakage, allowing fluid to seep out and accumulate in the retina. Without treatment, the pooling of this fluid stimulates a process similar to how scabs form, where the fluid is reorganized into a fibrous tissue that eventually forms scar tissue that causes permanent and significant declines in visual acuity. [20]
What are the different types of AMD? How can you tell them apart?
There are two types of AMD: nonexudative (or “dry”) and exudative (or “wet”) AMD. Drusen, geographic atrophy, and CNV are all features of AMD that can be used to distinguish between different types of the disease.
Nonexudative (‘Dry’) AMD
Nonexudative AMD has many names: non-neovascular AMD (meaning without new blood vessel formation), atrophic AMD (meaning without nourishment or without development), and most commonly, dry AMD, which refers to the lack of choroidal neovascularization in this form of AMD. All of these names describe different aspects of the condition. In general, dry AMD is characterized by the formation of many intermediate to large-sized drusen and changes in the pigmentation, or coloration, of the retina. [12, 19, 20] Notably, nonexudative AMD is characterized by a lack of CNV – in other words, there are no new blood vessels in dry AMD. Instead, the formation of drusen is associated with the degeneration of the macula.
Dry AMD accounts for roughly 85-90% of all AMD cases, and in its early stages, there may be no symptoms or visual changes. [21] However, as it progresses, the center of a patient’s visual field may gradually over months to years become blurry and distorted. If the disease continues to late disease stages, it can either become advanced dry AMD, which is characterized by geographic atrophy and severe declines in visual acuity, or it can become the second type of AMD, called wet AMD.
Exudative AMD
Exudative or ‘wet’ AMD is an advanced disease stage and generally the most severe form of AMD. While patients with wet AMD make up only 10-15% of all diagnosed cases of AMD, the condition accounts for 80% of all instances of severe vision loss and legal blindness (visual acuity less than 20/200 in both eyes) among AMD patients. [19, 22] Unlike dry AMD, wet AMD is characterized by the presence of CNV, or new blood vessels in the choroid. These vessels are very fragile and leak blood and fluid, which is sometimes referred to as ‘exudates,’ in the retina. That is why this form of AMD is called exudative AMD.
These new vessels can invade Bruch’s membrane, the RPE, and even the subretinal space. [12,19, 20] The resulting fluid and blood leakage can lead to fibrosis. Without treatment, this will eventually lead to significant decline in vision due to photoreceptor death. Once fibrosis forms, the resulting vision loss may be permanent.
How can my eyecare provider tell how much my AMD has progressed?
The clinical features of AMD, such as the presence of hard vs. soft drusen, geographic atrophy, and CNV, in addition to the type of condition, i.e. nonexudative vs exudative AMD, are all features that clinicians can use to determine how far a patient’s AMD has progressed.
The Age-Related Eye Disease Study (AREDS) and the Age-Related Eye Disease Study 2 (AREDS 2), were clinical trials conducted by the National Eye Institute (part of the National Institutes of Health) to learn more about AMD. [23] The researchers were able to use the aforementioned features to develop a classification system for the different stages of the disease. AMD is generally categorized into four categories.
How is AMD classified?
AREDS CLASSIFICATION GUIDELINES
CATEGORY 1: ESSENTIALLY NO AMD
A patient can have some small drusen and still be classified as category 1, as the development of a few drusen is a normal part of the aging process. As long as there are no more than 5 small drusen, the patient can be considered AMD-free.
CATEGORY 2: MILD AMD
At this stage, the patient has more than 5 small drusen or one medium-sized drusen and/or pigmentary, or coloration, changes observable in the retina
CATEGORY 3: INTERMEDIATE AMD
The patient has a few medium-sized drusen, and/or one large drusen, and/or geographic atrophy outside the center of vision.
CATEGORY 4: ADVANCED AMD
At this stage, the patient has atrophy, or break down, of photoreceptors or RPE resulting in geographic atrophy in the central macula (for dry AMD) or CNV and exudate (for wet AMD).
Eyecare providers are able to use the AREDS classification system as a guide to help diagnose patients. However, they also rely on a variety of other tools to assist in making an accurate assessment of a patient’s condition.
What tools will my eyecare provider use to diagnose and manage my AMD?
The eye is a relatively small organ, and the retina is a confined, hard-to-reach area in the back of the eye. Fortunately, eyecare providers have a variety of tools at their disposal to assist in making the diagnosis of AMD. These can include slit-lamp examination, retinal imaging including fundus autofluorescence, optical coherence tomography, fluorescein angiography, and indocyanine green angiography.
Fundoscopy
The word fundoscopy in this context refers to the process of an ophthalmologist examining the back of the eye. The root ‘fund-’ is derived from ‘fundus,’ which is a word that refers to the part of an organ that lies opposite of its opening. In this case, the opening of the eye is the pupil, and opposite of the pupil is the retina. The suffix ‘-oscopy’ means examination. Therefore, a fundoscopy is an examination of the back of the eye. This is usually done with the pupils dilated to get a good view of the retina.
Slit Lamp Examination
Typically, an ophthalmologist will use a machine called a slit lamp to perform the examination. A slit lamp is essentially a large microscope that allows an eyecare provider to see all of the structures of the eye. The eyecare provider (or a member of his or her team) will generally dilate your eyes with eye drops prior to examining you, and once the exam starts, a bright light will be shone into the eye. This may be slightly uncomfortable, but it is normal to experience some discomfort, since the dilating drops prevent the pupil from constricting in response to light as it normally would do.
When ophthalmologists examine the retina in the context of AMD, they are looking for any abnormalities that might be associated with the condition. Specifically, the presence of drusen, a hallmark of AMD. Pigmentary changes, or changes in the normal coloration of the retina, may be detectable as well. Exudate, or fluid from leaky vessels, may also be visible. These are all signs that a patient may have AMD, in which case the physician will likely use imaging techniques to confirm the diagnosis.
Fundus Autofluorescence
Fundus autofluorescence is an imaging procedure that takes advantage of the naturally occurring fluorescent properties of molecules in RPE cells. These molecules, called lipofuscin, are found in the RPE and they automatically begin to fluoresce when exposed to lights of certain wavelengths. [24] This natural property can be utilized for imaging purposes.
In AMD, hard drusen will often appear as bright white spots on fundus autofluorescence. This is because RPE cells that are undergoing degeneration are less efficient at removing and breaking down cellular debris from photoreceptors, leading to the accumulation of lipofuscin. Areas with more lipofuscin will fluoresce brighter than areas with less lipofuscin. As the RPE cells begin to die, the hyper-reflective white spots diminish in brightness until they eventually appear dark. At this point, the area has progressed to geographic atrophy. [12]
Fundus autofluorescence is one of the main tools for monitoring the progression of intermediate to advanced dry AMD and changes in geographic atrophy over time. The light emitted by the machine can be very bright and can be uncomfortable for some patients, although the imaging usually does not take long to perform, generally lasting only a few minutes.
Optical Coherence Tomography (OCT)
Optical coherence tomography (OCT) is an imaging modality that allows cross sectional images of the retina layers to be acquired non-invasively. In other words, rather than looking directly at the retina, the images appear as if the retina were sliced down the middle and examined on its side, enabling each cell layer to be viewed clearly. This allows for the retinal layers to be visualized in a way that normally would not be possible with fundoscopy or fundus autofluorescence. The presence, location, and size of drusen can be determined based on OCT, as well as the severity of fluid buildup within the retina, under the RPE or under the retina.
The machine works by shining light onto the retina, detecting any light that bounces back into the machine from the retina, and interpreting the returning light as an image. [24] The light that is projected onto the retina is in the infrared spectrum, so it is not visible to the naked eye. Thus, this imaging does not require any bright light and is a relatively comfortable procedure for patients, lasting only a few minutes depending on how many scans are required. Because of its high-quality, fast, and informative images, OCT has become a mainstay in ophthalmology practices and is the primary tool physicians use to monitor the progression of AMD.
Fluorescein and Indocyanine Green Angiography
Fluorescein angiography is the gold standard tool for diagnosing patients with wet AMD. Fluorescein sodium is a salt dye whose molecules excite in response to blue light illumination, causing them to fluoresce. [24] This dye is injected into a vein in the patient’s arm, after which they are imaged. The dye quickly travels through the bloodstream and into the eye, where it can reveal new vessel growth and leakage. Indocyanine green angiography works similarly to fluorescein angiography, but it uses a different dye that is better at being visualized through fluid, pigment, hemorrhage, etc. as well as highlighting choroidal issues.
What are the risk factors for developing AMD?
There are a number of risk factors that make certain individuals more prone to developing AMD. These include age, genetics, race, smoking status, and cardiovascular health.
Age
The greatest risk factor for developing AMD is increased age. [26-28] According to AREDS, the prevalence of individuals with category 3 AMD was 5.4% in individuals age 60-64 years of age, 10.2% in those 70-74 years, and 23.6% in individuals over the age of 80 in the US based on data from the 2000 US census. [12] Thus, as age increases, so does the risk of developing AMD.
Genetics
The genetic component of AMD development and progression is believed to play a considerable role in the condition. In fact, a family history of AMD is the second greatest risk factor for developing AMD. [12] Close relatives are three times more likely to develop wet AMD than individuals without any close affected relatives. [29, 30] Also, in studies of identical twins, if one twin developed AMD, there was a 90% chance that the other would too. This data is highly suggestive of a genetic influence in the onset of AMD. [31]
For a long time, researchers tried to find the ‘AMD gene,’ or a gene which, if mutated, could cause AMD. The search was fruitless until 2005, when a pioneering discovery revealed that a mutation in the gene encoding complement factor H (CFH) is involved in AMD onset. [20] Complement factor H plays a vital role in inflammation and immunity. Having a strong immune response can be good when faced with an infection, but an overactive immune system can begin to target the body’s own cells, leading to cell death. Mutations in CFH are believed to be responsible for up to 60-70% of the heritability of the condition. [32] In addition, there are multiple other gene mutations that are associated with an increased risk of developing AMD. Thus, genetic screenings to test for mutations in this gene may be an option to discuss with your eyecare provider, especially if you have an extensive family history of AMD.
Race
Based on the Baltimore Eye Study, Caucasians and Asians are the most susceptible to developing AMD, while it occurs less frequently among African American and Latino patients. [33] While African Americans and Latinos may develop early or intermediate AMD, they are less likely than Caucasians and Asians to progress to advanced disease stages or to develop blindness due to AMD.
Smoking Status
Smoking is the greatest modifiable risk factor for AMD onset and progression. In the AREDS study, researchers found that patients who had smoked in the past, even if they were not currently smoking, had twice the risk of developing wet AMD compared to individuals who had never smoked. Additionally, individuals who were category 1 on the severity scale but who smoked 20 or more cigarettes a day had twice the risk of developing AMD compared to individuals who had never smoked. [34] Both prior and current smokers typically develop AMD 5 to 10 years before non-smokers. [12] This data suggests that having a past smoking history or being an active smoker plays a significant role in the onset and progression of AMD.
Cardiovascular Health
Because wet AMD involves the growth of abnormal blood vessels, scientists have theorized that this process may be linked to poor cardiovascular health. While cardiovascular disease itself has not been confirmed as a definitive, independent risk factor for developing AMD, hypertension, or high blood pressure, has been widely implicated in AMD onset. [12] Other studies have suggested that obesity may also be linked to AMD, [35] while still others have demonstrated that AMD may be a side effect of other systemic, underlying health problems. Thus, overall health status may also affect the propensity of an individual to develop AMD.
What can I do to reduce my risk of developing AMD or to keep my AMD from getting worse?
While some of the aforementioned risk factors are out of your control (genetics, race), some of them are up to you to control. Below are several tips for improving your ocular health:
- Stop smoking.
- Eat a healthy diet full of dark-green leafy vegetables.
- Exercise regularly and maintain a healthy weight.
- Maintain healthy cholesterol and blood pressure levels. Get checked regularly to make sure you’re healthy.
- Schedule regular appointments with your ophthalmologist.
- Take a dietary supplement based on the AREDS clinical trials if you have AMD or are looking to maintain eye health earlier.
What is the prognosis? What are my treatment options?
Fortunately, the prognosis for patients with AMD continues to improve. Approximately 10-15 years ago, the advent of intravitreal injections (injections directly into the eye) of anti-vascular endothelial growth factor (VEGF) agents (medications which reduce the formation of new blood vessels and leakage from these blood vessels) has dramatically changed the prognosis for patients with AMD. Intravitreal injections have become a mainstay of treatment for wet AMD, and most patients with wet AMD are treated regularly with intravitreal injection therapy.
Unfortunately, there is no cure yet for AMD, but there are multiple new promising areas of treatment. Our hope is that this disease will continue to become less and less debilitating over time.
References:
[1] Wong WL, Su X, Li X, Cheung CM, Klein R, Cheng CY, Wong TY. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health. 2014;2(2):e106-116. https://www.ncbi.nlm.nih.gov/pubmed/25104651
[2] Gheorghe A, Mahdi L, Musat O. Age-Related Macular Degeneration. Rom J Ophthalmol. 2015;59(2):74-77. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5712933/
[3] Chen Y, Bedell M, Zhang K. Age-related macular degeneration: genetic and environmental factors of disease. Mol Interv. 2010;10(5):271-281. https://www.ncbi.nlm.nih.gov/pubmed/21045241
[4] Regatieri CV, Branchini L, Duker JS. The role of spectral-domain OCT in the diagnosis and management of neovascular age-related macular degeneration. Ophthalmic Surg Lasers Imaging. 2011;42 Suppl:S56-66. https://www.ncbi.nlm.nih.gov/pubmed/21790112
[5] Grossniklaus HE, Geisert EE, Nickerson JM. Introduction to the Retina. Prog Mol Biol Transl Sci. 2015;134:383-396. https://www.ncbi.nlm.nih.gov/pubmed/26310166
[6] Schulte D, Bumsted-O’Brien KM. Molecular mechanisms of vertebrate retina development: implications for ganglion cell and photoreceptor patterning. Brain Res. 2008;1192:151-164. https://www.ncbi.nlm.nih.gov/pubmed/17553468
[7] Wong-Riley MT. Energy metabolism of the visual system. Eye Brain. 2010;2:99-116. https://www.ncbi.nlm.nih.gov/pubmed/23226947
[8] Ting AY, Lee TK, MacDonald IM. Genetics of age-related macular degeneration. Curr Opin Ophthalmol. 2009;20(5):369-376. https://www.ncbi.nlm.nih.gov/pubmed/19587596
[9] Winkler BS. An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: renewal as a surrogate antioxidant. Invest Ophthalmol Vis Sci. 2008;49(8):3259-3261. https://www.ncbi.nlm.nih.gov/pubmed/18660422
[10] Mazzoni F, Safa H, Finnemann SC. Understanding photoreceptor outer segment phagocytosis: use and utility of RPE cells in culture. Exp Eye Res. 2014;126:51-60. https://www.ncbi.nlm.nih.gov/pubmed/24780752
[11] Holtkamp GM, Kijlstra A, Peek R, de Vos AF. Retinal pigment epithelium-immune system interactions: cytokine production and cytokine-induced changes. Prog Retin Eye Res. 2001;20(1):29-48. https://www.ncbi.nlm.nih.gov/pubmed/11070367
[12] Ambati J, Ambati BK, Yoo SH, Ianchulev S, Adamis AP. Age-related macular degeneration: etiology, pathogenesis, and therapeutic strategies. Surv Ophthalmol. 2003;48(3):257-293. https://www.ncbi.nlm.nih.gov/pubmed/12745003
[13] Bourla DH, Young TA. Age-related macular degeneration: a practical approach to a challenging disease. J Am Geriatr Soc. 2006;54(7):1130-1135. https://www.ncbi.nlm.nih.gov/pubmed/16866687
[14] Madonna RJ. Age-related Macular Degeneration Clinical Eye and Vision Care. 1997;9(2):65-129.
[15] Jager RD, Mieler WF, Miller JW. Age-related macular degeneration. N Engl J Med. 2008;358(24):2606-2617. https://www.ncbi.nlm.nih.gov/pubmed/18550876
[16] In: Dale Purves GJA, David Fitzpatrick, Lawrence C Katz, Anthony-Samuel LaMantia, James O McNamara, S Mark Williams, ed. Neuroscience, 2nd Edition: Sinauer Associates; 2001. https://www.ncbi.nlm.nih.gov/books/NBK10799/
[17] Wang S, Koster KM, He Y, Zhou Q. miRNAs as potential therapeutic targets for age-related macular degeneration. Future Med Chem. 2012;4(3):277-287. https://www.ncbi.nlm.nih.gov/pubmed/22393936
[18] Prasad PS, Schwartz SD, Hubschman JP. Age-related macular degeneration: current and novel therapies. Maturitas. 2010;66(1):46-50. https://www.ncbi.nlm.nih.gov/pubmed/20219298
[19] Cheung LK, Eaton A. Age-related macular degeneration. Pharmacotherapy. 2013;33(8):838-855. https://www.ncbi.nlm.nih.gov/pubmed/23580402
[20] Nowak JZ. AMD–the retinal disease with an unprecised etiopathogenesis: in search of effective therapeutics. Acta Pol Pharm. 2014;71(6):900-916. https://www.ncbi.nlm.nih.gov/pubmed/25745762
[21] Seddon JM, Chen CA. The epidemiology of age-related macular degeneration. Int Ophthalmol Clin. 2004;44(4):17-39. https://www.ncbi.nlm.nih.gov/pubmed/15577562
[22] Ferris FL, 3rd, Fine SL, Hyman L. Age-related macular degeneration and blindness due to neovascular maculopathy. Arch Ophthalmol. 1984;102(11):1640-1642. https://www.ncbi.nlm.nih.gov/pubmed/6208888
[23] Ferris FL, Davis MD, Clemons TE, Lee LY, Chew EY, Lindblad AS, Milton RC, Bressler SB, Klein R, Age-Related Eye Disease Study Research G. A simplified severity scale for age-related macular degeneration: AREDS Report No. 18. Arch Ophthalmol. 2005;123(11):1570-1574. https://www.ncbi.nlm.nih.gov/pubmed/16286620
[24] Yonekawa Y, Kim IK. Clinical characteristics and current treatment of age-related macular degeneration. Cold Spring Harb Perspect Med. 2014;5(1):a017178. https://www.ncbi.nlm.nih.gov/pubmed/25280900
[25] Madhusudhan S, Beare N. Wide-field fluorescein angiography in wet age-related macular degeneration. ScientificWorldJournal. 2014;2014:536161. https://www.ncbi.nlm.nih.gov/pubmed/25379537
[26] Klein R, Klein BE, Tomany SC, Meuer SM, Huang GH. Ten-year incidence and progression of age-related maculopathy: The Beaver Dam eye study. Ophthalmology. 2002;109(10):1767-1779. https://www.ncbi.nlm.nih.gov/pubmed/12359593
[27] Mitchell P, Wang JJ, Foran S, Smith W. Five-year incidence of age-related maculopathy lesions: the Blue Mountains Eye Study. Ophthalmology. 2002;109(6):1092-1097. https://www.ncbi.nlm.nih.gov/pubmed/9022098
[28] Friedman DS, O’Colmain BJ, Munoz B, Tomany SC, McCarty C, de Jong PT, Nemesure B, Mitchell P, Kempen J, Eye Diseases Prevalence Research G. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122(4):564-572. https://www.ncbi.nlm.nih.gov/pubmed/15078675
[29] Klaver CC, Wolfs RC, Assink JJ, van Duijn CM, Hofman A, de Jong PT. Genetic risk of age-related maculopathy. Population-based familial aggregation study. Arch Ophthalmol. 1998;116(12):1646-1651. https://www.ncbi.nlm.nih.gov/pubmed/9869796
[30] Klein ML, Mauldin WM, Stoumbos VD. Heredity and age-related macular degeneration. Observations in monozygotic twins. Arch Ophthalmol. 1994;112(7):932-937. https://www.ncbi.nlm.nih.gov/pubmed/8031273
[31] Meyers SM, Greene T, Gutman FA. A twin study of age-related macular degeneration. Am J Ophthalmol. 1995;120(6):757-766. https://www.ncbi.nlm.nih.gov/pubmed/8540549
[32] Fritsche LG, Fariss RN, Stambolian D, Abecasis GR, Curcio CA, Swaroop A. Age-related macular degeneration: genetics and biology coming together. Annu Rev Genomics Hum Genet. 2014;15:151-171. https://www.ncbi.nlm.nih.gov/pubmed/24773320
[33] Sommer A, Tielsch JM, Katz J, Quigley HA, Gottsch JD, Javitt JC, Martone JF, Royall RM, Witt KA, Ezrine S. Racial differences in the cause-specific prevalence of blindness in east Baltimore. N Engl J Med. 1991;325(20):1412-1417. https://www.ncbi.nlm.nih.gov/pubmed/1922252
[34] Christen WG, Glynn RJ, Manson JE, Ajani UA, Buring JE. A prospective study of cigarette smoking and risk of age-related macular degeneration in men. JAMA. 1996;276(14):1147-1151. https://www.ncbi.nlm.nih.gov/pubmed/8827967
[35] Rastogi N, Smith RT. Association of age-related macular degeneration and reticular macular disease with cardiovascular disease. Surv Ophthalmol. 2016;61(4):422-433. https://www.ncbi.nlm.nih.gov/pubmed/26518628
[36] Age-Related Eye Disease Study 2 Research G. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: the Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA. 2013;309(19):2005-2015. https://www.ncbi.nlm.nih.gov/pubmed/23644932
[37] Sharon D Solomon JSS. Age-Related Macular Degeneration. Vol 32012:81-92.
[38] Verteporfin In Photodynamic Therapy Study G. Verteporfin therapy of subfoveal choroidal neovascularization in age-related macular degeneration: two-year results of a randomized clinical trial including lesions with occult with no classic choroidal neovascularization–verteporfin in photodynamic therapy report 2. Am J Ophthalmol. 2001;131(5):541-560. https://www.ncbi.nlm.nih.gov/pubmed/11336929
[39] Photodynamic therapy of subfoveal choroidal neovascularization in age-related macular degeneration with verteporfin: one-year results of 2 randomized clinical trials–TAP report. Treatment of age-related macular degeneration with photodynamic therapy (TAP) Study Group. Arch Ophthalmol. 1999;117(10):1329-1345. https://www.ncbi.nlm.nih.gov/pubmed/10532441
[40] Senger DR, Galli SJ, Dvorak AM, Perruzzi CA, Harvey VS, Dvorak HF. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983;219(4587):983-985. https://www.ncbi.nlm.nih.gov/pubmed/6823562
[41] Gragoudas ES, Adamis AP, Cunningham ET, Jr., Feinsod M, Guyer DR, Group VISiONCT. Pegaptanib for neovascular age-related macular degeneration. N Engl J Med. 2004;351(27):2805-2816. https://www.ncbi.nlm.nih.gov/pubmed/15625332
[42] Rosenfeld PJ, Brown DM, Heier JS, Boyer DS, Kaiser PK, Chung CY, Kim RY, Group MS. Ranibizumab for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1419-1431. https://www.ncbi.nlm.nih.gov/pubmed/17021318
[43] Brown DM, Kaiser PK, Michels M, Soubrane G, Heier JS, Kim RY, Sy JP, Schneider S, Group AS. Ranibizumab versus verteporfin for neovascular age-related macular degeneration. N Engl J Med. 2006;355(14):1432-1444. https://www.ncbi.nlm.nih.gov/pubmed/17021319
[44] Berrocal MH, Lewis ML, Flynn HW, Jr. Variations in the clinical course of submacular hemorrhage. Am J Ophthalmol. 1996;122(4):486-493. https://www.ncbi.nlm.nih.gov/pubmed/8862044
[45] Hawkins BS, Bressler NM, Miskala PH, Bressler SB, Holekamp NM, Marsh MJ, Redford M, Schwartz SD, Sternberg P, Jr., Thomas MA, Wilson DJ, Submacular Surgery Trials Research G. Surgery for subfoveal choroidal neovascularization in age-related macular degeneration: ophthalmic findings: SST report no. 11. Ophthalmology. 2004;111(11):1967-1980. https://www.ncbi.nlm.nih.gov/pubmed/15522362
[46] Borrillo JL, Regillo CD. Treatment of subretinal hemorrhages with tissue plasminogen activator. Curr Opin Ophthalmol. 2001;12(3):207-211. https://www.ncbi.nlm.nih.gov/pubmed/11389348
[47] Casten RJ, Rovner BW, Tasman W. Age-related macular degeneration and depression: a review of recent research. Curr Opin Ophthalmol. 2004;15(3):181-183. https://www.ncbi.nlm.nih.gov/pubmed/15118503
[48] Decensi A, Robertson C, Guerrieri-Gonzaga A, Serrano D, Cazzaniga M, Mora S, Gulisano M, Johansson H, Galimberti V, Cassano E, Moroni SM, Formelli F, Lien EA, Pelosi G, Johnson KA, Bonanni B. Randomized double-blind 2 x 2 trial of low-dose tamoxifen and fenretinide for breast cancer prevention in high-risk premenopausal women. J Clin Oncol. 2009;27(23):3749-3756. https://www.ncbi.nlm.nih.gov/pubmed/19597031
[49] Lotery A, Trump D. Progress in defining the molecular biology of age related macular degeneration. Hum Genet. 2007;122(3-4):219-236. https://www.ncbi.nlm.nih.gov/pubmed/17659362
[50] Girmens JF, Sahel JA, Marazova K. Dry age-related macular degeneration: A currently unmet clinical need. Intractable Rare Dis Res. 2012;1(3):103-114. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4204600/
[51] Emerich DF, Thanos CG. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther. 2008;10(5):506-515. https://www.ncbi.nlm.nih.gov/pubmed/18830926
[52] Falkner-Radler CI, Krebs I, Glittenberg C, Povazay B, Drexler W, Graf A, Binder S. Human retinal pigment epithelium (RPE) transplantation: outcome after autologous RPE-choroid sheet and RPE cell-suspension in a randomised clinical study. Br J Ophthalmol. 2011;95(3):370-375. https://www.ncbi.nlm.nih.gov/pubmed/20610478
[53] Chaum E, Hatton MP. Gene therapy for genetic and acquired retinal diseases. Surv Ophthalmol. 2002;47(5):449-469. https://www.ncbi.nlm.nih.gov/pubmed/12431694
[54] Reich SJ, Fosnot J, Kuroki A, Tang W, Yang X, Maguire AM, Bennett J, Tolentino MJ. Small interfering RNA (siRNA) targeting VEGF effectively inhibits ocular neovascularization in a mouse model. Mol Vis. 2003;9:210-216. https://www.ncbi.nlm.nih.gov/pubmed/12789138
[55] Holash J, Davis S, Papadopoulos N, Croll SD, Ho L, Russell M, Boland P, Leidich R, Hylton D, Burova E, Ioffe E, Huang T, Radziejewski C, Bailey K, Fandl JP, Daly T, Wiegand SJ, Yancopoulos GD, Rudge JS. VEGF-Trap: a VEGF blocker with potent antitumor effects. Proc Natl Acad Sci U S A. 2002;99(17):11393-11398. https://www.ncbi.nlm.nih.gov/pubmed/12177445